Drug-induced liver injury (Drug-Induced Liver Injury, referred to as DILI) refers to diseases caused by liver drug toxicity damage or allergic reaction when a therapeutic dose of drug is applied. According to the statistics of the World Health Organization, drug-induced liver injury has risen to the fifth place in the global cause of death. There are currently more than 1,100 drugs that may cause liver damage, most of which are unpredictable liver damage, with an incidence rate of 1 / 100,000 to 10,000. With the rapid development of China's pharmaceutical industry, new drugs, health products, and diet pills have emerged in recent years, and the use of drugs in clinical practice is also increasingly common. Drug-induced liver injury is not only the object of epidemiological research, but also one of the important contents of clinical medical safety research.
According to the results of the International Medical Organization Committee CIOMS proposed in 1989 and updated by the US FDA in 2001, the clear definition of liver injury is: Alanine Aminotransferase (hereinafter referred to as ALT) exceeds the upper limit of normal value (Upper Limit of Normal, hereinafter referred to as ULN 3 times, alkaline phosphatase (Alkaline Phosphatase, hereinafter referred to as ALP) more than 2 times ULN, or bilirubin (Bilirubin, hereinafter referred to as BILI) more than 2 times the upper limit of normal value, accompanied by an increase in ALT or ALP . In addition, Aspartate Aminotransferase (AST) is also an important basis for evaluating liver injury and liver toxicity. It can be seen that in the clinical diagnosis of drug-induced liver injury, quantitative interpretation and comprehensive analysis of various laboratory test results are essential.
The American scholar Dr. Hyman Zimmerman clearly put forward the famous Hy's Law (Hy's Law), that is, when drug-induced liver injury occurs ALT> = 3ULN and BILI> = 2, it usually indicates a poor prognosis. In the absence of biliary obstruction, even if the relevant drugs are stopped, the mortality rate may still reach 10% or more. This rule has become the reference standard for evaluating liver toxicity in the FDA's new drug research and development evaluation, and is also one of the medical judgment criteria trusted by many medical researchers.
In order to further simplify the safety review of clinical trial data and shorten the expensive and cumbersome drug development process, more and more drug regulatory agency reviewers, CRO company statisticians, hospital doctors, and epidemiologists have begun to use new graphics Tools to assist in the clinical diagnosis of drug-induced liver injury.
The following are two typical application cases, which are shared with interested readers. They are based on clinical trial research data and are implemented using SAS's new generation data analysis platform for life sciences-JMP Clinical software. JMP Clinical can To help shorten the expensive and cumbersome drug development process, it has been adopted by FDA and SFDA.
The first graph is a schematic diagram of Hy's rules, where the red dots represent subjects taking new drugs, the blue triangles represent subjects taking placebo; the X axis represents the logarithm of ALT / ULN with 2 as the base, The Y axis represents the logarithmic value of BILI / ULN with 2 as the base. According to Hy's rule, we added a reference line on the X axis of 1.58496 and the Y axis of 1.00 respectively. In this way, the entire coordinate system is divided into four quadrants. Among them, the two indexes of alanine aminotransferase and bilirubin in the first quadrant are very high, which meets the reference standard of Hy's rule and is the place where serious adverse events occur; the alanine aminotransferase and bile in the third quadrant Both indexes of erythromycin are relatively low and relatively safe; one of the two indexes, alanine aminotransferase and bilirubin, in the second and fourth quadrants is very high, and the other is low, but it also deserves attention.
In this way, we can intuitively find that the drug-induced liver injury caused by the new drug NIC.15 is very serious, because there are subjects appearing in the first quadrant in the figure, and the subjects appearing have taken This new drug, and most of the subjects who appeared in the second and fourth quadrants also took this new drug. The test results of placebo subjects were relatively normal, and most of them were concentrated in the third quadrant.
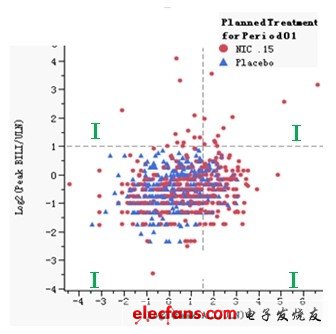
Figure 1: Schematic diagram of Hy's rules based on JMP Clinical software.
The second graph is the change trend graph of the test indicators of the subjects. The red dots represent the subjects taking new drugs, and the blue dots represent the subjects taking placebo; the X axis represents alanine aminotransfer The enzyme test results, the Y axis represents the bilirubin test results. From the perspective of medical statistics, this is a dynamic scatterplot that incorporates time dimension information, which can reflect the changes in the pathological characteristics of the subject during the medication and the new drug group and comfort in the form of digital animation Comparison of changes in agent groups.
In this way, we can intuitively find that: in many test populations, four new drug subjects numbered 141063, 321012, 282031, and 141058 had serious adverse reactions after taking NIC.15. The laboratory indexes related to liver injury have risen significantly, and it is necessary to pay attention to it. The drug should be stopped immediately and other suitable treatments should be taken. At the same time, this phenomenon once again shows that the new drug NIC.15 will cause serious drug-induced liver injury, and the existence of its clinical safety risks is beyond doubt.
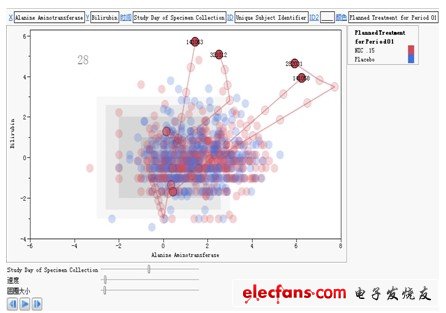
Figure 2: The trend graph of subjects' test indicators based on JMP Clinical software.
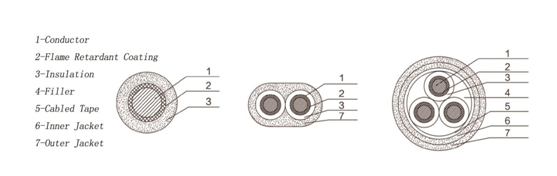
PVC Fire resistant cables(Wires) are coated with a self developed fire retardant (FR) pvc compound that halts the spread of fire even in extreme temperature cases. The compound also offers a high dielectric strength and high insulation. Fire-resistant cable can still ensure the normal operation of lines within certain time in case of combustion. It is different from the ordinary flame retardant cable in that in the event of a fire, it may continue electricity transmission. The use of this product will allow high-rise buildings, subways, power plants and other major occasions to have better fire safety and fire rescue capability.

Advantages:
- High Insulation
- Longer flex life
- Excellent electrical properties
- Chemical & acid resistance
- Large tensile strength
- Good softness
- Excellent elasticity and stickiness
Standard:
GB/T19216.21
Rated Voltage:
450/750V
Application:
This cable is designed for areas where the integrity of the electrical circuit is critical in maintaining power supply. Applications can be found in emergency lightings, control and power circuits, power stations, fire alarm systems, underground tunnels, communication systems, sewage treatment plants, lifts, escalators and high-rise buildings
Welcome to visit our factory to learn more about us. If you have any questions, please feel free to contact us.
PVC Insulated Fire Resistant Wire
PVC Insulated Fire Resistant Wire,Fire Resistant Cable,Heat Resistant Electrical Wire,Fire Resistant PVC Insulated Copper Wire
Smartell Technology Co.,Ltd , http://www.liencable.com